Lithium-ion batteries, which won the Nobel Prize for Chemistry in 2019, are essential for powering everything from smartphones to electric vehicles.
Composition and Standards
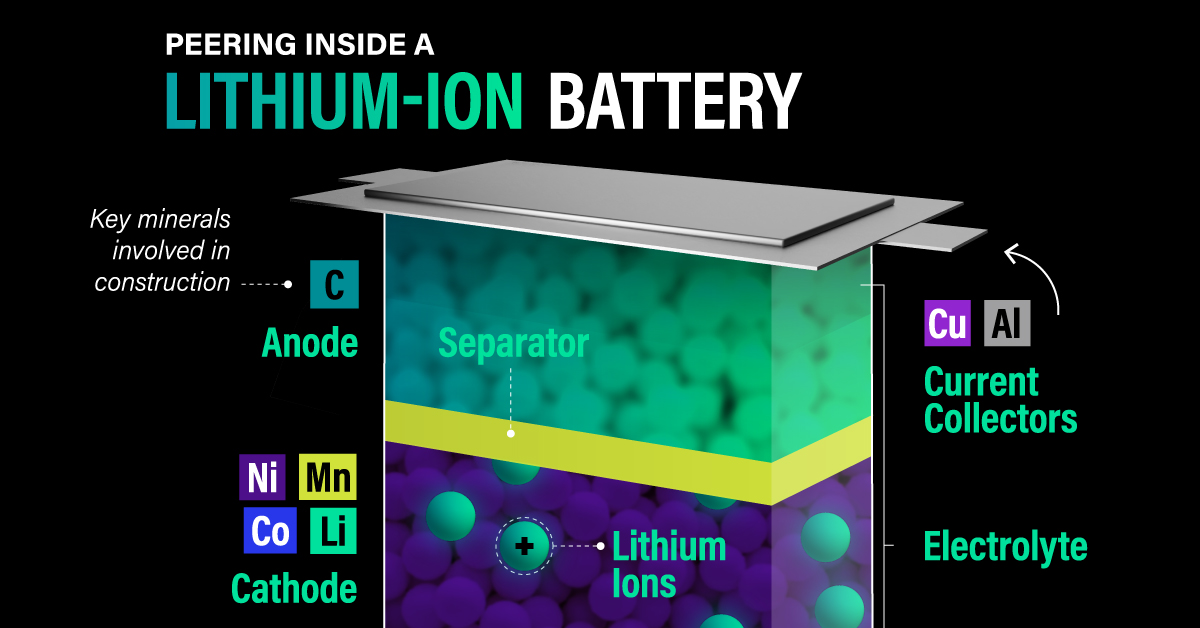
Lithium-ion batteries vary by region, with NMC/NMCA standards in Europe and North America offering higher energy density and LFP standards in China providing a lower-cost alternative.
Here is the typical mineral composition of a lithium-ion battery, accounting for these two main cathode types:
| Material | % of Construction |
|---|---|
| Nickel (Ni) | 4% |
| Manganese (Mn) | 5% |
| Lithium (Li) | 7% |
| Cobalt (Co) | 7% |
| Copper (Cu) | 10% |
| Aluminum (Al) | 15% |
| Graphite (C) | 16% |
| Other Materials | 36% |
The lithium content is expressed as the percentage of lithium carbonate equivalent (LCE) in the battery. Typically, 1g of lithium metal corresponds to 5.17g of LCE.
Functionality
Lithium-ion batteries operate by collecting current during charging, where a graphite anode attracts and holds lithium ions. Emerging research suggests that using a thin layer of pure lithium instead of graphite can nearly double the energy density.
During discharge, the cathode draws the stored lithium ions, funneling them to another current collector. The anode and cathode are separated and suspended in a medium that allows ions to flow easily, preventing contact between the two.
Future of Energy Storage
Lithium, despite being only 7% of a battery’s weight on average, is crucial for the manufacture of lithium-ion batteries. Recognizing its importance, the U.S. Geological Survey has classified lithium as one of the 35 critical minerals for the U.S. economy.
Refining lithium more efficiently is vital to meeting the demand for next-generation lithium-ion batteries. EnergyX is at the forefront of this effort, developing advanced lithium metal batteries with longer cycle life, greater energy density, and faster charging times, thus driving the clean energy transition.